The Role of Stem Cell Therapy in Treating Corneal Disease
Corneal dystrophies are a group of inherited conditions that cause structural problems in the cornea, the eye’s clear outer window. Over time, these conditions can lead to a buildup of cloudy material or changes in the cornea's shape, causing blurred vision and, in advanced cases, significant sight loss. This progressive degeneration is why finding effective, accessible treatments is a major focus in ophthalmology.
The Limits of Current Treatments
Many of these conditions, particularly keratoconus, disproportionately affect younger individuals. Keratoconus causes the cornea to thin and bulge into a cone shape, leading to distorted vision that cannot be corrected with standard glasses. Its onset often occurs during puberty, disrupting education and careers during a critical life stage. For decades, the main solution for advanced corneal disease has been a corneal transplant, which replaces damaged tissue with a healthy donor cornea. While sight-restoring, this invasive surgery carries risks, requires a long recovery, and includes the lifelong potential for immune system rejection, which can lead to graft failure and the need for another transplant.
Compounding these challenges is a severe global shortage of donor tissue. It is estimated that for every 70 people who need a transplant, only one donor cornea is available, leaving millions worldwide with impaired vision. This scarcity, combined with a lack of eye banking infrastructure and specialized surgeons in many parts of the world, makes the standard of care inaccessible for a vast number of patients who could otherwise benefit.
A Regenerative Solution: How Stem Cells Rebuild the Cornea
In response to these challenges, a revolutionary approach is emerging from regenerative medicine. Instead of replacing damaged corneal tissue, this new paradigm focuses on regenerating it from within using the body’s own powerful repair mechanisms. This groundbreaking therapy harnesses the potential of stem cells to halt disease progression and restore the cornea’s natural structure.
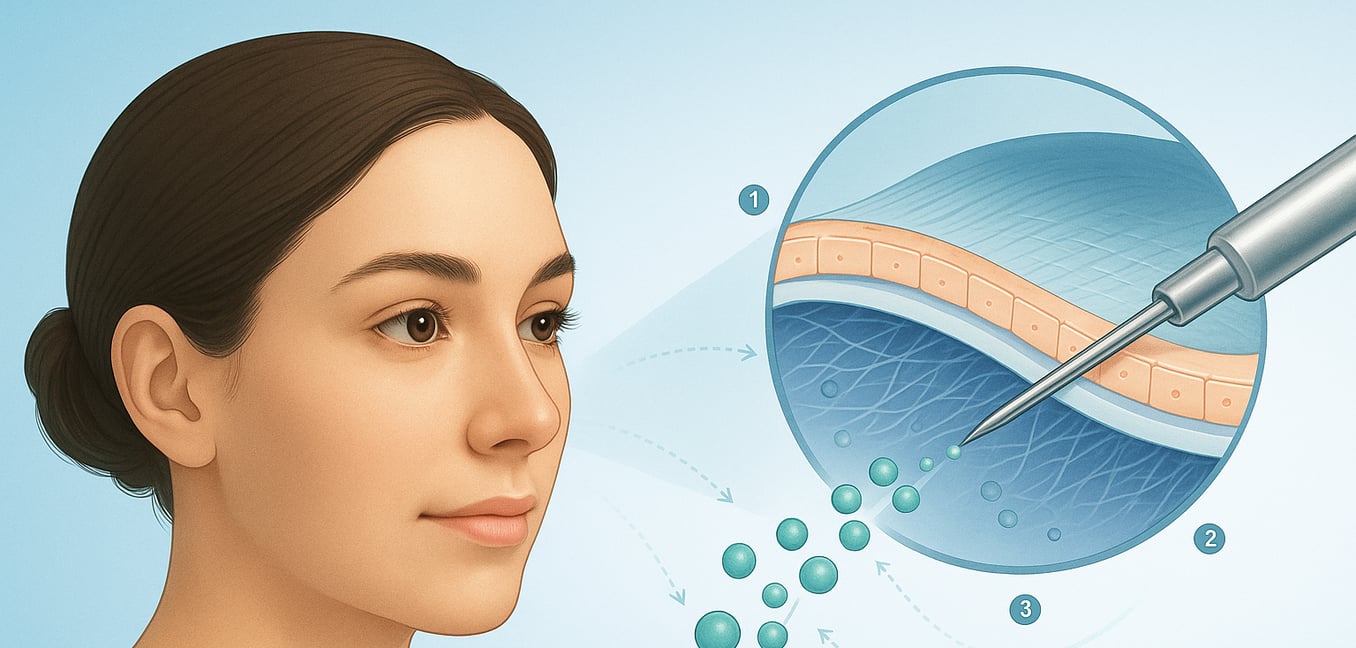
The treatment uses a patient’s own adult stem cells, specifically mesenchymal stem cells (MSCs) harvested from fat tissue. Because these cells come from the individual’s own body, the immune system recognizes them as friendly and does not attack them. This type of self-sourced procedure, known as an autologous treatment, eliminates the risk of rejection seen with donor tissue and sidesteps the severe global shortage of donor corneas.
The therapeutic process involves carefully injecting these prepared stem cells directly into the corneal stroma, the middle layer that is weakened in conditions like keratoconus. Guided by the surrounding environment, these versatile cells transform into keratocytes—the cornea's native cells responsible for maintaining its strength and clarity. This cellular therapy effectively repopulates the thinning tissue, prompting the cornea to produce its own collagen and biologically revitalize itself, thereby strengthening its structure without a full transplant.
These stem cells do more than just rebuild. They also have natural healing properties that calm inflammation, prevent scarring, and regulate the local immune response. This creates the ideal environment for the cornea to heal and can improve its optical clarity, offering a dual action of both regenerating tissue and clearing up existing cloudiness.
Targeting Specific Corneal Layers
The cornea is a sophisticated structure with distinct layers, and researchers are developing specialized stem cell therapies designed to repair the exact layer affected by disease. This targeted strategy promises more precise and effective treatments.
Repairing the Outer Surface: Epithelial Cell Therapy
For damage to the cornea's outermost protective layer, the epithelium, a therapy known as CALEC (cultivated autologous limbal epithelial cells) shows remarkable promise. Ideal for patients with limbal stem cell deficiency, often caused by chemical burns, the procedure involves taking a tiny biopsy of stem cells from the patient's healthy eye. These cells are expanded in a lab into a new sheet of tissue and then transplanted onto the injured eye. Because the cells are the patient's own, there is no rejection risk, and trials have shown high success rates in restoring a stable, healthy corneal surface.
Restoring the Inner Pump: Endothelial Cell Injections
Deeper inside, the delicate endothelial layer acts as the cornea’s pump, preventing it from swelling. When this layer fails, as in Fuchs’ dystrophy, injectable cell therapies offer a minimally invasive solution. Companies like Aurion Biotech and Emmecell are pioneering techniques where healthy endothelial cells, grown from a single donor cornea, are simply injected into the eye. These cells are encouraged to attach and form a new, functional layer, sometimes with the help of a ROCK inhibitor or magnetic nanoparticles to guide them. This approach could replace complex transplants, requires a much shorter recovery, and dramatically addresses the donor shortage, as cells from one donor cornea could potentially treat 100 or more patients.
The Next Frontier: Universal Donor Cells
Looking ahead, the next step is developing treatments for patients with damage to both eyes who lack a healthy eye to draw cells from. This involves allogeneic therapies that use cells from a cadaver donor. While this would expand access, it reintroduces the challenge of managing immune rejection. The ultimate goal is the use of induced pluripotent stem cells (iPSCs), which can be generated from adult skin or blood cells and then guided to become any type of corneal cell needed. This technology could one day provide a limitless, off-the-shelf supply of custom-matched cells, completely revolutionizing treatment.
From Lab to Clinic: The Global Progress of Corneal Cell Therapy
The journey from scientific discovery to an available patient treatment is long, but for corneal cell therapies, that transition is happening now. These advanced treatments are moving out of the laboratory and into clinical settings, marking a pivotal moment in the fight against corneal blindness.
Landmark Approval in Japan
A significant breakthrough has occurred in Japan, where Aurion Biotech received the world's first regulatory approval for an allogeneic cell therapy to treat bullous keratopathy. Developed from the research of Professor Shigeru Kinoshita, the therapy uses cultured endothelial cells from a single donor. This approval by Japan’s medical device agency confirms the therapy's safety and effectiveness, representing a historic leap from experimental research to standard medical practice.
Advancing Clinical Trials in the United States
The United States is also a hub of clinical progress. Aurion Biotech has initiated Phase 1/2 trials for its therapy, while Emmecell has completed Phase 1 safety studies for its magnetic cell delivery system. Furthermore, Cellusion Inc. has received an orphan drug designation from the FDA for its iPSC-derived treatment, helping to fast-track its development. These trials are essential for gathering the data needed to bring these treatments to American patients.
Pioneering Stromal Regeneration in Spain
In Spain, a major milestone has been achieved in treating keratoconus. Led by Dr. Jorge Alió, a project completed the first implant of stem cells from adipose tissue to regenerate the corneal stroma. The culmination of 14 years of research, the technique aims to biologically revitalize the cornea, stopping the disease and offering a crucial alternative to transplantation for patients with advanced keratoconus.
The Future Horizon: Standardizing and Scaling Up Treatment
As these groundbreaking therapies move toward real-world practice, the focus is shifting to making them widely available and creating reliable treatment protocols. This next phase is crucial for ensuring the promise of regenerative medicine can be realized by patients everywhere.
Goal 1: Simplifying Delivery for Broader Access
A key goal is to simplify the delivery of these therapies so they are not limited to a few highly specialized surgeons. Some injectable treatments are designed to be straightforward enough for a general ophthalmologist to perform in an office setting, representing a monumental shift in accessibility. This would remove the need for an operating room and bring treatment to patients in underserved areas, potentially at an earlier stage of disease.
Goal 2: Overcoming Manufacturing and Logistics
The transition from treating hundreds of trial participants to potentially thousands of patients hinges on solving complex manufacturing challenges. Creating a consistent supply of therapeutic cells requires costly "Good Manufacturing Practice" (GMP) facilities and highly controlled processes. A major question is whether to centralize production in a few super-labs or create multiple regional centers. Furthermore, the logistics of shipping living cells under precise conditions is a significant hurdle for reliable distribution.
Goal 3: Navigating Global Regulation and Guidelines
While approval in one country is a major victory, each new region presents its own set of regulatory hurdles. The process in the United States through the FDA is known for its rigor, and European agencies have their own unique requirements. The future will require not only navigating these pathways but also establishing clear, evidence-based clinical guidelines to define which patients are the best candidates and ensure these powerful new treatments are used safely and effectively.