For so many families in our community, the journey with a rare genetic disease starts with a single, tiny error in the body’s instruction manual—our DNA. We know that understanding the science behind these conditions can be one of the first steps toward feeling empowered. For decades, treatments have focused on managing symptoms rather than addressing the source of the problem. But today, we want to walk with you through a development that represents a new chapter of hope, a story of incredible scientific progress that gets to the very heart of these conditions.
We’re hearing more about a groundbreaking technology called in vivo base editing. We know these scientific terms can feel intimidating, so our goal here is to break it down together. This is a story about moving from making big changes to our DNA to making incredibly precise ones. It’s about a new kind of "molecular pencil" that offers a gentler, more targeted approach to correcting the original genetic typo.[1]
This is a deep dive into the science, the hope, and the realistic challenges of this new frontier. We believe that by understanding it, we can have more informed conversations, ask better questions, and march forward together with a clearer sense of what the future may hold.
From Molecular Scissors to a Genetic Pencil: The Evolution of Gene Editing
To understand the breakthrough of base editing, it helps to first look at the technology that came before it: CRISPR-Cas9. You may have heard of CRISPR described as “molecular scissors.”[1] It was a revolutionary discovery that gave scientists the ability to find a specific spot in our DNA and make a cut. This was a monumental leap forward, allowing researchers to “turn off” faulty genes. This technology is the foundation for the first-ever approved CRISPR-based therapy for sickle cell disease, Casgevy, where cells are taken out of the body, edited with these scissors, and then returned to the patient.
While incredibly powerful, the idea of making a double-strand cut in our DNA has always come with challenges. The cell’s natural repair process for such a cut can be a bit unpredictable, sometimes leading to unintended changes.[2] We know that for any therapy to be used widely, especially inside the body, safety is the absolute top priority.
This is where base editing comes in, representing the next step in this journey of progress. Developed by researchers, including a team led by Dr. David Liu at Harvard University and the Broad Institute, base editing was designed to be more like a “molecular pencil.”[3][4] It was created to make a precise change to a single letter of the DNA code without making a disruptive cut.[5][6]
Here’s how we can think about it working:
-
The Guide: A molecule called a guide RNA (gRNA) acts like a GPS, leading the base editor to the exact, specific "typo" in the billions of letters that make up our DNA.[1]
-
The Anchor: The base editor uses a deactivated CRISPR protein (often called a nickase or "dead" Cas9) as an anchor. It holds on to the DNA at the right spot but—and this is the key difference—it doesn’t make a double-strand cut.[5][7]
-
The Pencil: Fused to this anchor is a special enzyme (a deaminase) that acts as the pencil. It performs a chemical reaction that effectively erases the incorrect DNA letter and replaces it with the correct one.[7][8]
The beauty of this approach is its precision and its gentle nature. By avoiding a cut, it sidesteps many of the concerns associated with the original “molecular scissors,” offering a potentially safer way to correct the fundamental genetic errors that cause so many rare diseases.[1][9]
The Delivery Challenge: Getting the Pencil to the Right Page
Having an amazing "genetic pencil" is one thing, but getting it to the right cells deep inside the body is one of the biggest challenges in all of gene therapy.[2][10] This is the "in vivo" part of the puzzle—making the edits within the living body. For the many conditions that affect organs like the liver, brain, or muscles, this is the only way a genetic therapy can work.
Scientists are working tirelessly on this delivery problem, and two main approaches have emerged as the front-runners. Think of them as two different types of microscopic delivery trucks:
-
Viral Vectors (like AAVs): These are essentially the shells of viruses that have been hollowed out and re-engineered so they can’t cause disease. Instead, they are filled with the genetic instructions for making the base editor.
-
The Upside: Viruses are naturally very good at getting into our cells, and different types of Adeno-Associated Viruses (AAVs) are known to target specific tissues like the muscles or the eyes.[11] They have a long track record in gene therapy research.
-
The Challenges: We know that our bodies are designed to fight off viruses. This means that many people have pre-existing immunity that could stop the vector before it does its job.[12][13] This immune response also makes it difficult to give a second dose.[12] Furthermore, AAVs have a limited "cargo space" of about 4.7 kilobases, which can be a tight squeeze for the base editing machinery.[14][15]
-
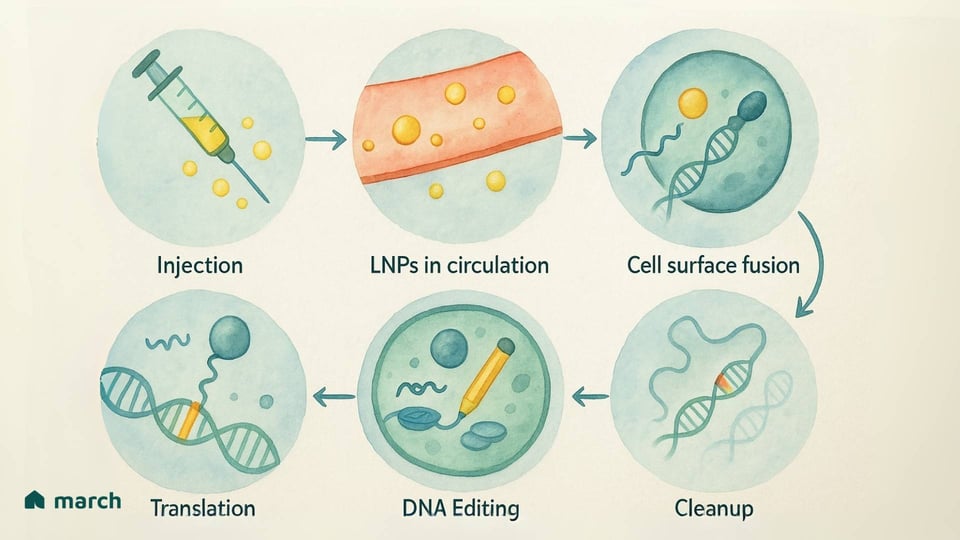
Lipid Nanoparticles (LNPs): You may have heard about these from the mRNA COVID-19 vaccines. LNPs are tiny, engineered bubbles of fat that can carry the genetic instructions (in the form of mRNA) for the base editor.[16][17]
-
The Upside: LNPs have a larger cargo space and are less likely to trigger a strong immune response, which means re-dosing might be possible.[2][16] A huge safety advantage is that they deliver temporary instructions. The cell makes the "pencil," it does its job, and then the instructions and the pencil are naturally cleared away. This short window of activity reduces the risk of any unwanted edits.[18]
-
The Challenges: Right now, when given through an IV, most LNPs are naturally taken up by the liver.[19][20] While this is perfect for treating liver diseases, researchers are working hard to engineer new LNPs that can be directed to other tissues throughout the body.[16]
The progress on these delivery systems is just as important as the editing tools themselves. Every step forward in making these "trucks" safer and more targeted is a step forward for our community.
A Landmark Moment: The First Human to Receive In Vivo Base Editing
For years, this technology was the subject of hopeful research in labs. But recently, we witnessed a landmark moment that moved it from theory into reality. The story of K.J. Muldoon, a baby born with a severe and life-threatening rare genetic disease, has been a source of incredible inspiration.
K.J. was diagnosed with carbamoyl-phosphate synthetase 1 (CPS1) deficiency, a rare metabolic disorder where the liver can’t process ammonia, a toxic byproduct of breaking down protein.[21][22] This condition can be devastating, with an estimated 50% mortality rate in early infancy.[23] We know the fear and uncertainty that come with a diagnosis like this.
But K.J.’s specific condition was caused by a single-letter typo in his DNA—a perfect candidate for base editing. In a remarkable effort, a personalized therapy was designed just for him in only six months.[21] He received an intravenous infusion of an LNP—the tiny fat bubble—carrying the mRNA instructions for a base editor "pencil" designed to fix his exact mutation.[24][25]
The early results have been a source of profound hope:[23]
-
K.J. began to tolerate more protein in his diet.
-
The dose of the medications he needed to control his ammonia levels was significantly reduced.
-
He started to reach his developmental milestones.
-
Most powerfully, he was able to fight off two common childhood viruses without spiraling into a life-threatening crisis, something that would have been almost certain before the therapy.
We must walk this path with both hope and realism. This was a therapy for one brave child, and we still need to see how long the correction will last.[23] But this single story is a powerful proof-of-concept. It shows that this technology is possible and has the potential to change lives. It also opens the door to a future where therapies could be tailored to an individual’s unique genetic makeup.[24]
This isn’t just one story. Research is actively underway for other conditions. In lab models of Progeria, a disease of premature aging, base editing has corrected the genetic typo and dramatically extended the lifespan of mice. Clinical trials using base editing are also underway for conditions like high cholesterol (led by Verve Therapeutics) and Alpha-1 Antitrypsin Deficiency (led by Beam Therapeutics).[26][27] This wave of research is driven by the needs of many different patient communities, and every success builds momentum for the next.
Marching Forward Together: The Journey Ahead
The dawn of in vivo base editing is incredibly exciting, but as a community, we know that progress must be made responsibly and with transparency. There are still significant challenges to overcome, and it’s important we talk about them openly.
Safety is Always First: The top priority is ensuring the "pencil" only edits the intended DNA letter and nothing else. Scientists have developed rigorous methods to check for these "off-target" edits and are constantly working to make the tools even more precise.[9][28]
Understanding Long-Term Effects: For any therapy that makes a permanent change, we need to follow patients for a very long time to ensure the benefits are durable and that no unexpected issues arise down the road.[10][29]
The Challenge of Access: We know that these advanced therapies are currently incredibly complex and expensive to make, with some gene therapies costing over $2 million per treatment.[30][31][32] As a community, it is vital that we advocate for a future where these life-changing treatments are accessible to everyone who needs them, regardless of where they live or what resources they have.[33] This is a conversation we must have now, at the very beginning.
The field is not standing still. Scientists are already developing the next generation of tools, like prime editing, another innovation from Dr. Liu's lab that works like a "search and replace" function for DNA and can fix even more types of genetic errors, including small insertions and deletions.[34][35][36][37][38][39]
As we watch this new frontier of medicine unfold, we are committed to providing you with clear, trustworthy information. We will celebrate the breakthroughs and be honest about the challenges. We will continue to build a community where we can learn, share, and support one another. Your insights, your experiences, and your hope are what fuel this forward march. On this journey of discovery, you are not alone.
For a quick overview, check out our new podcast episode, exploring the history and science of this therapy.
https://youtu.be/-aVHZciahbw
Sources
[7] Menichiello, T. (2023, September 27). Base Editing And Prime Editing: How They're Changing Gene Therapy. Cell & Gene.
[30] Rueda, J., de Miguel Beriain, I., & Montoliu, L. (2024). Affordable Pricing of CRISPR Treatments is a Pressing Ethical Imperative. CRISPR Journal.
[1] BioTechniques. (2017, November 1). Molecular Pencils Rewrite Bases.
[40] Verve Therapeutics. (2025). VERVE-102. Retrieved from vervetx.com.
[31] Glick, B. (2023, December 8). Pricey new gene therapies for sickle cell pose access test. BioPharma Dive.
[33] CRISPR Therapeutics Canada. (2025, January 28). The Real Cost of CRISPR Treatment: What Canadian Patients Need to Know.
[8] Genethique. (2017, November 2). ABE (adenine base editor): a "base editor" to complete the CRISPR tool kit.
[21] Technology Networks. (2025, June 27). How the First Customized Gene Therapy Was Created in 6 Months.
[2] Open Access Journals. (n.d.). Problems Faced by Physicians in Gene Therapy. Journal of Gene Therapy.
[41] Rare Disease Advisor. (2025, April 15). Verve Therapeutics Reports Promising Results for PCSK9 Gene Editing in HeFH.
[12] Biocompare. (2025, January 2). Overcoming Challenges in AAV- and rAAV-based Gene Therapies.
[32] Rueda, J., de Miguel Beriain, I., & Montoliu, L. (2024, October 14). Affordable Pricing of CRISPR Treatments is a Pressing Ethical Imperative. ResearchGate.
[10] Consensus. (n.d.). What are the challenges of developing gene therapies for genetic disorders?.
[14] Form Bio. (n.d.). The Role of AAV in Gene Therapy.
[22] Sanders, R. (2025, May 15). Infant born with deadly disease now thriving thanks to customized CRISPR treatment six months after birth. Berkeley News.
[5] Ma, Y., et al. (2023). Precise genome editing with base editors. Journal of Genetics and Genomics.
[34] Howard Hughes Medical Institute. (2020, June 12). Precision Genome Editing Enters the Modern Era.
[9] MedNexus. (2024). Targeted Gene Therapy: Promises and Challenges in Disease Management. Journal of Bio-X Research.
[15] BioInnovatise. (2024, December 9). AAV Packaging Capacity Resources.
[24] Ahn, N. (2025, May 22). Penn Med, CHOP researchers treat infant with world's first personalized gene-editing therapy. The Daily Pennsylvanian.
[28] Frontiers in Pediatrics. (2024). Advances and challenges in gene therapy strategies for pediatric cancer: a comprehensive update.
[35] Rett Syndrome Research Trust. (n.d.). Prime Editing.
[29] Patsnap. (2025, March 20). What are the ethical challenges in gene therapy?.
[26] Taylor, N.P. (2025, May 5). 'Safer' CRISPR: Base Editing Breaks Through in the Clinic as Beam, Verve Advance. BioSpace.
[36] Cross, R. (2019, December 2). Introducing CRISPR 3.0. C&EN Global Enterprise.
[23] Garcia de Jesus, E. (2025, May 28). Personalized gene editing saved a baby, but the tech's future is uncertain. Science News.
[37] Synthego. (n.d.). Prime Editing as a Precision Gene Editing Tool.
[6] SCMP. (2023, May 31). Chinese scientists develop new gene-editing tool that differs in approach to CRISPR-Cas9.
[27] DeFeudis, N. (2025, March 11). Beam shares promising first clinical data on gene editing treatment for a lung and liver disease. Endpoints News.
[42] Wu, G. (2025, March 24). Verve gets FDA green light to expand base editing trial into US. BioPharma Dive.
[3] Broad Institute. (2025, April 5). David Liu receives Breakthrough Prize in Life Sciences.
[4] Howard Hughes Medical Institute. (2025, April 5). David Liu Awarded Breakthrough Prize.
[43] Rueda, J., de Miguel Beriain, I., & Montoliu, L. (2024). Affordable Pricing of CRISPR Treatments is a Pressing Ethical Imperative. Digital CSIC.
[13] High, K. A., & Roncarolo, M. G. (2019). Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Molecular Therapy.
[38] O'Hanlon Cohrt, K. (2023, December 4). Explainer: What Is Prime Editing and What Is It Used For?. CRISPR Medicine News.
[44] O'Hanlon Cohrt, K. (2025, May 14). The Latest Clinical Trial Updates from CRISPR Medicine News.
[16] GenScript. (2024, October 15). Lipid Nanoparticles: A Breakthrough in CRISPR Delivery Systems.
[39] Davies, H., & Auguste, A. (2023). Base and Prime Genome Editing in Precision Oncology. Cancer Research.
[11] Revvity. (n.d.). AAV vectors: challenges and solutions for gene therapy manufacturing.
[45] Verve Therapeutics. (2025, May 14). Verve Therapeutics Announces Pipeline Progress and Reports First Quarter 2025 Financial Results.
[46] CRISPR Medicine News. (2025, March 11). Beam Therapeutics Reports Clinical Proof-Of-Concept Data for BEAM-302 in Alpha-1 Antitrypsin Deficiency Trial.
[47] Verve Therapeutics. (2025, April 11). Verve Therapeutics Receives U.S. FDA Fast Track Designation for VERVE-102, an In Vivo Base Editing Medicine Targeting PCSK9.
[48] Beam Therapeutics. (2025, March 10). Beam Therapeutics Announces Positive Initial Data for BEAM-302 in the Phase 1/2 Trial in Alpha-1 Antitrypsin Deficiency (AATD), Demonstrating First Ever Clinical Genetic Correction of a Disease-causing Mutation.
[25] Children's Hospital of Philadelphia. (2025, May 15). World's First Patient Treated with Personalized CRISPR Gene Editing Therapy at Children's Hospital of Philadelphia.
[17] ACS Publications. (2022, May 20). Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components.
[18] PubMed Central (PMC). (n.d.). Lipid nanoparticles: The game-changer in CRISPR-Cas9 genome editing.
[49] Promega Connections. (2025, May 28). Base Editing Brilliance: David Liu's Breakthrough Prize and Its Impact.
[19] Nano Magazine. (2022, December 19). Lipid nanoparticle-mediated delivery of CRISPR components for neuronal genome editing.
[20] ACS Nanoscience Au. (2023, March 30). Recent Advances in Site-Specific Lipid Nanoparticles for mRNA Delivery.