Nutrition and Diet for Glutaryl-CoA Dehydrogenase Deficiency
Glutaryl-CoA Dehydrogenase (GCDH) deficiency, also known as Glutaric Acidemia Type I (GA-I), is an inherited metabolic disorder. It impairs the body's ability to process the amino acids lysine, hydroxylysine, and tryptophan due to a faulty GCDH enzyme. This leads to a harmful buildup of intermediate substances.
Understanding the basics of GA-I is key to managing its nutritional aspects:
- GCDH Enzyme Function: This enzyme is vital for breaking down lysine, hydroxylysine, and tryptophan. When deficient, this process is disrupted, causing specific metabolic byproducts to accumulate.
- Genetic Inheritance: GA-I is an autosomal recessive disorder. A child must inherit two copies of the mutated GCDH gene, one from each parent, to have the condition. Carriers, with one mutated gene, are usually unaffected.
- Metabolic Impact and Toxin Buildup: The enzyme deficiency causes substances like glutaric acid, 3-hydroxyglutaric acid, and glutarylcarnitine (C5DC) to collect in the blood, urine, and tissues. The brain, especially the basal ganglia (a region controlling movement), is highly susceptible to these accumulating waste products.
- Neurological Consequences: This toxic buildup can damage the brain, particularly during metabolic stress like illness or fasting. Potential issues include sudden episodes of brain dysfunction (encephalopathic crises), movement disorders such as dystonia (involuntary muscle contractions), seizures, and developmental delays. An unusually large head (macrocephaly) is a common early sign in infants.
The Importance of Early Diagnosis and Consistent Monitoring
Prompt identification and continuous management are crucial for GA-I due to the risk of severe neurological damage, especially in infancy and early childhood.
Key elements for effective management include:
- Newborn Screening (NBS): NBS is a critical tool, often detecting GA-I by identifying elevated glutarylcarnitine (C5DC) in dried blood spots before symptoms appear. This allows for swift confirmatory testing and early treatment, significantly reducing the risk of brain injury, including damage to the striatum (a key part of the brain involved in movement).
- Comprehensive Diagnostic Confirmation: An abnormal NBS result requires further tests. These usually involve analyzing urine for organic acids (especially increased 3-hydroxyglutaric acid) and detailed plasma acylcarnitine profiles. Genetic testing for GCDH gene mutations provides a definitive diagnosis.
- Lifelong Monitoring: Regular, lifelong monitoring is essential. This includes periodic checks of plasma amino acids (especially lysine), carnitine levels, and urine organic acids to assess dietary adherence and metabolic control. Consistent clinical follow-up tracks growth, neurodevelopment, and overall health, helping to optimize therapy and prevent crises.
Dietary Management: Restricting Lysine and Tryptophan
The primary dietary strategy for GA-I involves carefully controlling the intake of lysine, hydroxylysine, and tryptophan to minimize the buildup of harmful compounds.
This dietary approach focuses on:
- Targeting Precursor Amino Acids: The main goal is to restrict lysine and, to a lesser extent, tryptophan. These amino acids are the direct sources of substances like glutaric acid. Limiting their intake reduces the production of these compounds that can be harmful to the brain.
- Specialized Medical Foods and Arginine: A highly specialized diet, using lysine-free and often tryptophan-restricted medical foods, is necessary. These formulations provide essential nutrients without the problematic amino acids. They also often include supplemental arginine, which may compete with lysine for transport into the brain, potentially offering extra protection. The diet requires careful calculation of natural protein intake, guided by a metabolic dietitian.
L-Carnitine Supplementation: A Key Therapeutic Component
Building on its role as an essential partner in detoxification, L-carnitine supplementation is a cornerstone of GA-I management, working alongside dietary restrictions.
L-carnitine contributes in several ways:
- Aiding Detoxification: L-carnitine binds with toxic metabolites like glutaric acid, forming compounds such as glutarylcarnitine (C5DC). This process makes the toxins more water-soluble and easier for the kidneys to excrete.
- Preventing Secondary Carnitine Deficiency: The body's efforts to remove excess organic acids can deplete its carnitine stores. Supplementation replenishes these levels, ensuring carnitine is available for detoxification and other functions.
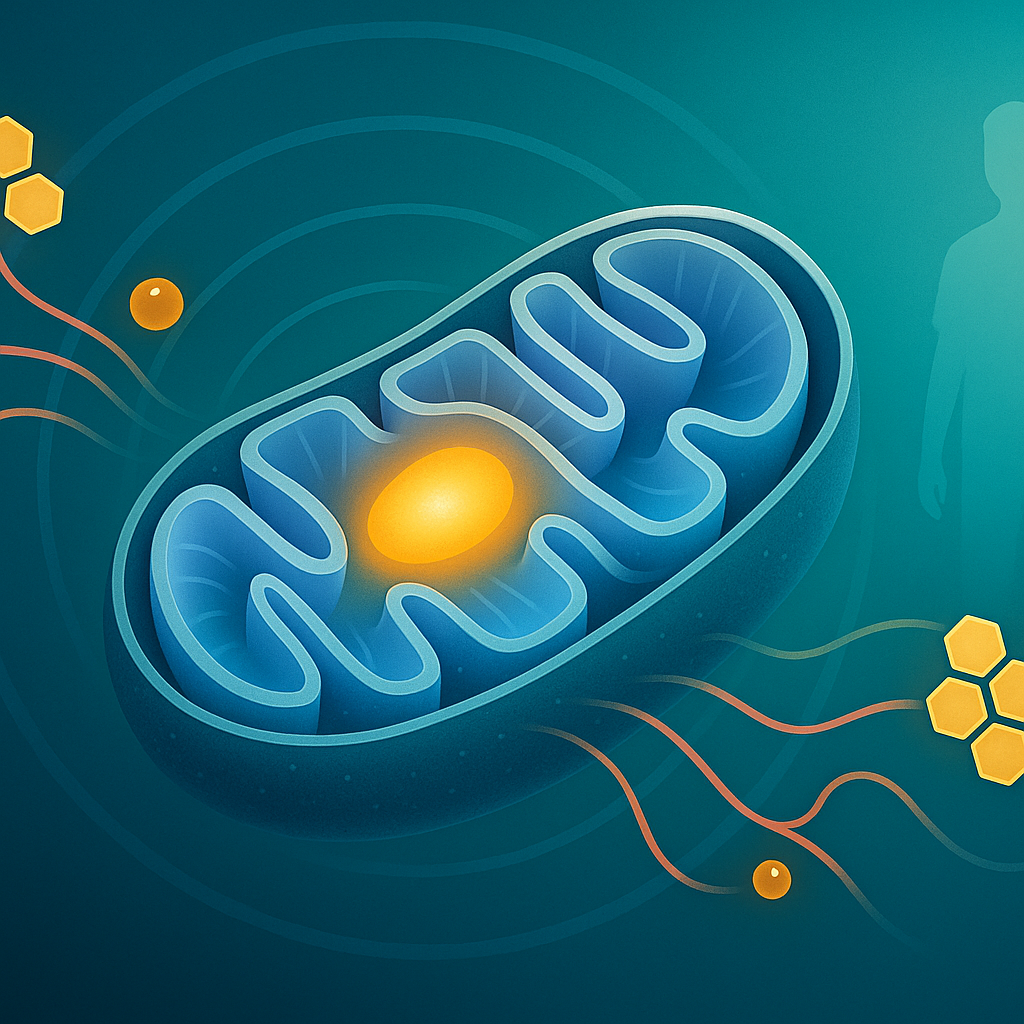
- Supporting Energy Metabolism: L-carnitine is crucial for energy production, especially from fats. It transports certain fats into the mitochondria – the powerhouses of our cells – where they are converted into ATP, the body's main energy source.
- Improving Muscle Function and Vitality: Adequate L-carnitine levels can improve muscle strength and tone, which may be concerns in GA-I. By aiding detoxification and energy production, it contributes to overall well-being and may reduce the severity of metabolic crises.
Additional Nutritional Support and Emergency Protocols
Comprehensive GA-I management includes strategies for illness and other specific nutritional considerations. Additional considerations for comprehensive care include:
- Emergency Protocols for Illness: Illnesses can trigger metabolic crises. Emergency plans involve increasing calorie intake (mainly from carbohydrates), temporarily reducing or stopping natural protein, and continuing lysine-free medical foods. Hydration, often with glucose-containing fluids, is vital. Families should have an 'emergency letter' from their metabolic team for hospital guidance.
- Riboflavin (Vitamin B2) Consideration: Riboflavin is sometimes used as it is a precursor to FAD, a cofactor for enzymes, with the hope of supporting any residual GCDH activity. While generally safe, its benefit for long-term neurological outcomes is not definitively proven, though it may lower some metabolic markers. Its use is individualized.