For so many in our community, the journey with synovial sarcoma begins not with clarity, but with a frustrating and often lengthy search for answers.[1] We know this path is often filled with uncertainty. Because it's a rare cancer, primarily affecting adolescents and young adults (the AYA population), its initial symptoms—often a slow-growing, painless lump—are frequently mistaken for something more common, like a sports injury or a cyst.[2][4] This can lead to a "diagnostic odyssey" that can last up to two years, a critical delay for an aggressive cancer.[1]
This diagnosis falls upon young people at a pivotal time in their lives, disrupting education, careers, and family planning. For decades, the road forward for those with advanced or metastatic disease has been daunting. Standard chemotherapy regimens, while important, have offered limited long-term success.[3] For adults diagnosed with metastatic synovial sarcoma, the five-year overall survival rate has been a grim 10%.[3] Second-line treatments like pazopanib or trabectedin, while providing options, typically offer modest benefits, with median overall survival hovering around 10 months.[5] There has been a profound, deeply felt unmet need for a true breakthrough.
But today, we want to walk with you through a story of scientific progress that offers a new kind of hope. It’s a therapy that doesn’t just fight the cancer, but teaches a patient’s own body how to do it. We’re talking about a landmark new treatment called afamitresgene autoleucel, or Tecelra.[11]
This is a deep dive into the science, the hope, and the realistic challenges of this new frontier. We believe that by understanding it, we can have more informed conversations with our care teams, ask better questions, and march forward together with a clearer sense of what the future may hold. This therapy is not just a new drug; it's a new chapter, one that gets to the very heart of what makes synovial sarcoma unique.
The Science: A Lock, a Key, and a Trained Soldier
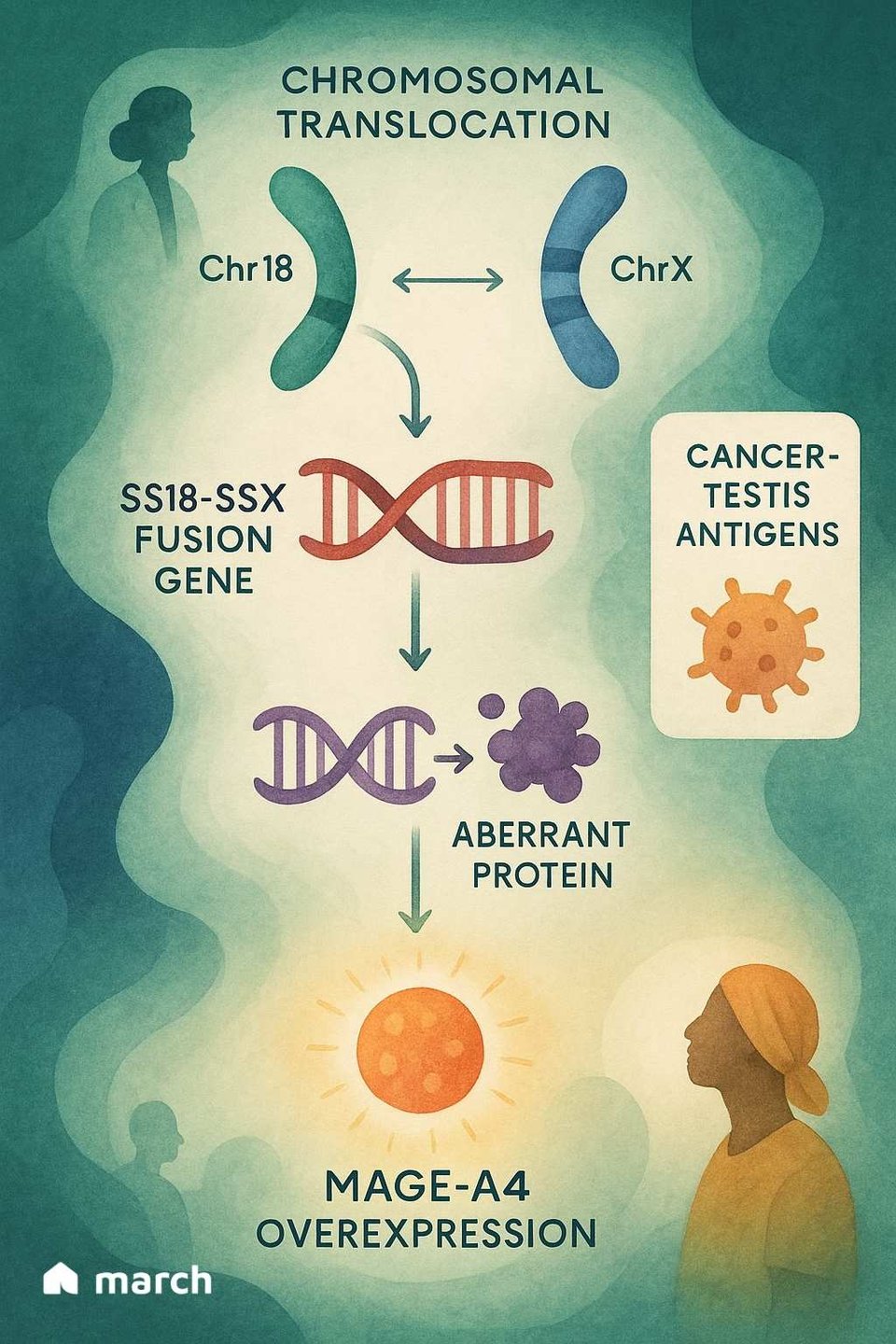
To truly grasp the importance of Tecelra, we have to start with the unique biology of synovial sarcoma itself. Unlike many cancers that have hundreds of different genetic mutations, synovial sarcoma is often "mutationally quiet."[4] Over 95% of cases are driven by a single, specific genetic event: a translocation where parts of chromosome 18 and chromosome X swap places.[6][7] This creates a powerful and destructive fusion gene called SS18-SSX.[6]
This SS18-SSX fusion protein doesn't cause cancer by itself; instead, it hijacks the cell's internal machinery that controls which genes get turned on or off.[6] One of the consequences of this genetic chaos is that the cancer cells begin to produce proteins that normal adult cells don't, including a protein called MAGE-A4.[8]
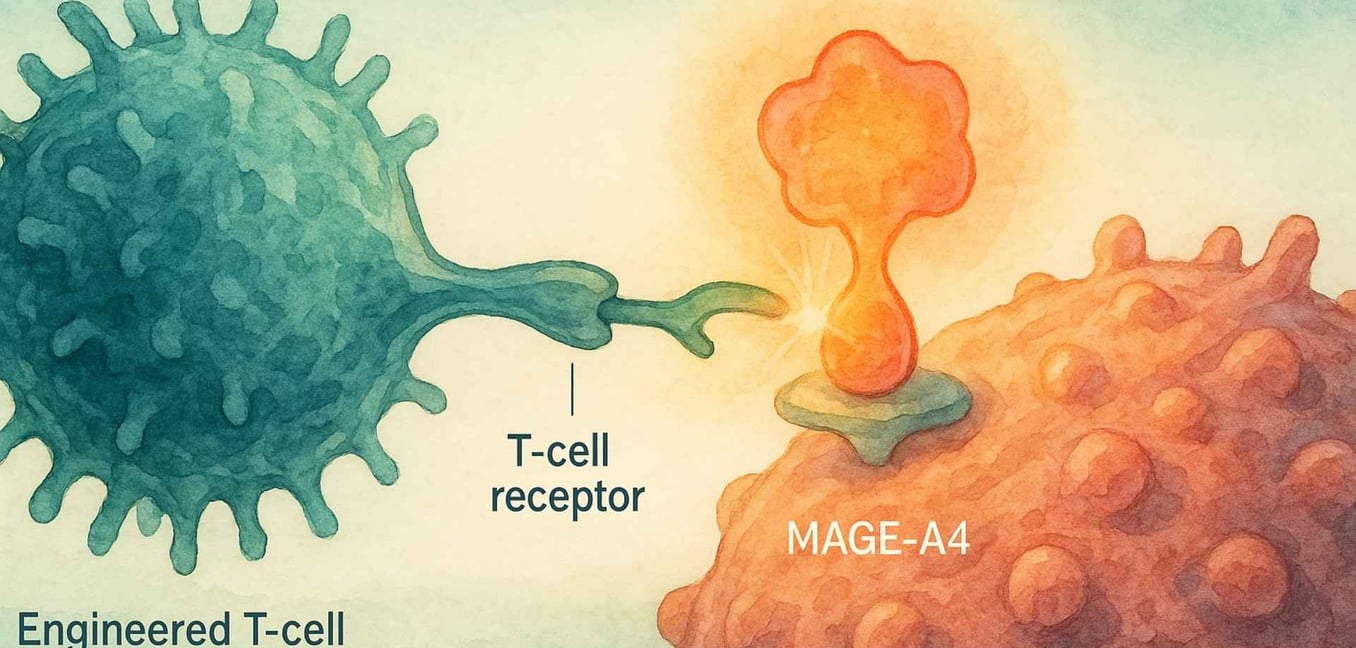
MAGE-A4 is a "cancer-testis antigen," meaning it's normally only found in immune-privileged sites like the testes.[9] When it appears on a sarcoma cell, it acts like a foreign flag, signaling to the immune system that something is wrong.[8] This is where Tecelra’s elegant strategy comes into play.
From CAR-T to TCR: Targeting the Untouchable
Many of us have heard of CAR-T therapy, which has been revolutionary for blood cancers.[10] CAR-T cells are engineered to recognize targets on the surface of cancer cells. However, the vast majority of cancer-driving proteins, like MAGE-A4, are inside the cell, making them invisible to CAR-T.[20]
This is where T-Cell Receptor (TCR) therapy comes in. All our cells use a system called the HLA complex to constantly show the immune system bits and pieces of the proteins inside them.[13] If a cell is cancerous, it will display fragments of cancer proteins on its surface. TCR T-cell therapy engineers a patient's T-cells to recognize these very specific fragments.[20]
Tecelra is the first FDA-approved TCR therapy for a solid tumor, a true landmark achievement.[11] It works because it solves two problems at once:
-
The Lock (The Target): It gives a patient's T-cells a new, high-affinity TCR that is expertly designed to find the MAGE-A4 protein fragment.[13]
-
The Key (The Handshake): It can only "see" this fragment if it's being presented by a specific HLA molecule, HLA-A*02.[13]
This two-part recognition system—the MAGE-A4 "lock" and the HLA-A*02 "key"—makes the therapy incredibly precise, but also highly personalized.[13] It will only work for patients who have both of these biological markers.[14]
The Tecelra Process: A Patient’s Journey to a Living Medicine
Understanding the science is one thing; knowing what the journey actually entails is another. The process of receiving Tecelra is intensive and requires a significant commitment from patients and their caregivers. It’s a multi-step path that transforms a patient's own cells into a powerful, living medicine.
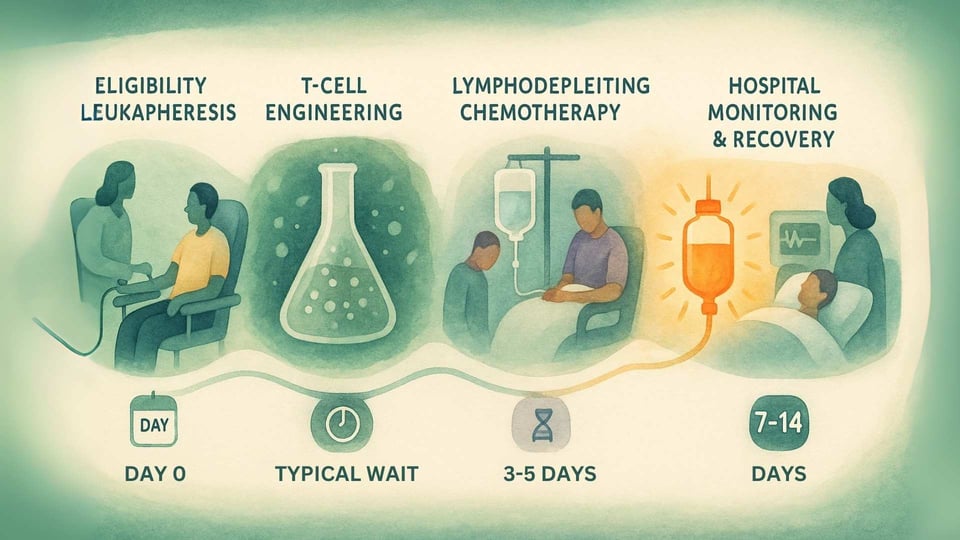
Step 1: Confirmation and Collection (Leukapheresis)
The journey begins with testing to ensure a patient is eligible. This involves a blood test to confirm they have the required HLA-A*02 genetic marker and a biopsy test of their tumor to confirm it expresses the MAGE-A4 protein.[14] Once eligibility is confirmed, the patient undergoes leukapheresis, a non-surgical procedure that filters T-cells from their blood.[12]
Step 2: The Manufacturing Wait
The collected T-cells are frozen and sent to a specialized manufacturing facility. Here, they undergo the genetic engineering process to become Tecelra.[13] This is a complex and careful process that, in the clinical trial, took a median of 40 days.[14] During this waiting period, a patient's doctor may recommend "bridging therapy," such as chemotherapy, to keep the cancer under control.[14]
Step 3: Preparing the Body (Lymphodepletion)
A few days before the infusion, the patient is admitted to the hospital to receive lymphodepleting chemotherapy (typically fludarabine and cyclophosphamide).[14] The goal of this chemotherapy is not to kill the cancer, but to temporarily reduce the number of existing immune cells in the body.[15] This creates a clear and welcoming environment for the new, engineered T-cells to engraft, expand, and persist for a long-lasting effect.[15]
Step 4: The Infusion (Day Zero)
After lymphodepletion, the patient receives their personalized Tecelra cells through a single intravenous (IV) infusion.[14] This moment is often called "Day Zero," marking the start of their new immune system's fight against the cancer.
Step 5: Intensive Monitoring and Support
The post-infusion period is the most critical. Because the therapy is designed to create a powerful immune response, patients must be monitored in the hospital for at least 7 days.[14] They are also advised to stay close to the specialized treatment center for at least 4 weeks. Recognizing the logistical and financial burden, the manufacturer provides a support program, AdaptimmuneAssist, which can help eligible patients with travel, lodging, and other out-of-pocket costs.[16]
A Story Told in Data: The Hope of the SPEARHEAD-1 Trial
For years, this remarkable science was a concept researched in laboratories. But the SPEARHEAD-1 clinical trial moved it from theory into reality, offering tangible hope to 44 people with advanced synovial sarcoma who had already been through a median of three prior lines of systemic therapy.[14][17] These were patients for whom standard options had stopped working. The results, published in the prestigious journal The Lancet, were a source of incredible encouragement.[14]
Let's look at the numbers, because they tell a powerful story of hope:
-
A Meaningful Response (ORR): In this heavily pre-treated group, 43% of patients saw their tumors shrink in response to the one-time infusion of Tecelra.[11][14] This is known as the overall response rate (ORR), and it's a significant figure where later-line therapies often have response rates well below 20%.
-
Durable, Lasting Benefits (DoR): For those who responded to the therapy, the effects were often lasting. The median duration of response (DoR) was 6.0 months.[14] Crucially, 39% of those who responded were still seeing that benefit one year later, suggesting a durable, long-term effect for a significant group.[14]
-
The "Tail on the Curve" (Overall Survival): Perhaps the most powerful finding was what happened to the patients who responded to Tecelra over the long term. For the entire group of synovial sarcoma patients, the median overall survival (OS) was approximately 17 months.[14] But for those whose cancer responded to the therapy, the median OS had not yet been reached, and an estimated 70% of these responders were still alive at the two-year mark.[14] This creates what researchers call a "tail on the survival curve." It suggests that for a subset of patients, this living medicine isn't just a temporary fix; it has the potential to provide a long-term, durable defense against the cancer, fundamentally changing their outlook.
For a community that has been waiting over a decade for a new therapeutic option, these results represent a monumental and emotional step forward. It is the kind of progress that shows science is listening, and that the needs of rare disease patients are driving innovation.
The Journey Ahead: Realism, Responsibility, and the Horizon
We walk this path with you, holding both hope and honesty. This is a powerful therapy, and it’s crucial to understand the significant side effects. Because it's designed to powerfully activate the immune system, Tecelra comes with a U.S. Boxed Warning for two main, potentially life-threatening toxicities:[11]
-
Cytokine Release Syndrome (CRS): This was the most common serious side effect, occurring in 75% of patients in the trial.[14] CRS is a systemic inflammatory response that can cause high fever, nausea, low blood pressure, and other symptoms. While most cases are mild to moderate and can be managed effectively with supportive care and medications, 2% of patients experienced severe (Grade 3 or higher) CRS.[14]
-
Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS): This is a rarer neurological side effect, seen in about 2% of patients at a low grade.[14]
These risks are precisely why Tecelra can only be administered at specialized, Authorized Treatment Centers with expertly trained teams.[18]
The arrival of Tecelra is truly a new dawn, but we know it's not the end of the journey. The conversation around advanced therapies must include the challenges of access and cost. With a reported list price of $727,000, ensuring equitable access is a major ethical and practical challenge for our healthcare system.[19] As advocates, we must continue to push for a future where every eligible person can benefit.
The story of Tecelra is about more than just a single drug. It's about the relentless march of science, and it opens the door to future innovation. Research is already underway exploring other targets, like the NY-ESO-1 antigen, and next-generation TCRs designed to be even more effective.[20]
As we watch this new frontier unfold, we are committed to providing you with clear, trustworthy information. Your insights, your experiences, and your hope are what fuel this forward march. On this journey of discovery, you are not alone.
Sources
-
Aytekin, M. N., et al. (2022). The "Great Masquerader" in Orthopedics: Synovial Sarcoma. Cureus.
-
Orphanet. (n.d.). Synovial sarcoma.
-
National Cancer Institute. (n.d.). Synovial Sarcoma Treatment (PDQ®)–Health Professional Version.
-
King, D.M., et al. (2020). A review of synovial sarcoma. Journal of the American Academy of Orthopaedic Surgeons.
-
Martin-Broto, J., et al. (2022). Pazopanib and Trabectedin for Patients with Metastatic Synovial Sarcoma: A Real-World Study. Cancers.
-
Lagarde, P., et al. (2016). The SS18-SSX fusion oncoprotein. The Journal of Pathology.
-
PathologyOutlines.com. (n.d.). Synovial sarcoma - Molecular.
-
Saito, T., et al. (2023). MAGE-A4 is a potential therapeutic target in synovial sarcoma. Oncology Reports.
-
Cancer Research Institute. (n.d.). Cancer-Testis Antigens.
-
National Cancer Institute. (n.d.). CAR T-Cell Therapy and Its Side Effects.
-
U.S. Food and Drug Administration. (2024). FDA grants accelerated approval to afamitresgene autoleucel.
-
National Cancer Institute. (n.d.). Leukapheresis. NCI Dictionary of Cancer Terms.
-
Adaptimmune. (n.d.). Our Technology.
-
D'Angelo, S. P., et al. (2024). Afamitresgene autoleucel for advanced synovial sarcoma (SPEARHEAD-1). The Lancet.
-
Jain, T., et al. (2019). CAR T-Cell Therapy: The Role of Lymphodepletion. The Hematologist.
-
Adaptimmune. (n.d.). AdaptimmuneAssist.
-
D'Angelo, S. P., et al. (2021). Afamitresgene autoleucel for advanced synovial sarcoma or myxoid/round cell liposarcoma. ASCO Post.
-
Adaptimmune. (n.d.). Authorized Treatment Centers.
-
Leo, L. & Sunny, S.E. (2024). US FDA approves Adaptimmune's gene therapy for rare cancer. Reuters.
-
Ma, S., et al. (2021). Current landscape of T-cell receptor-engineered T cell therapy for solid tumors. Journal of Hematology & Oncology.