For so many families in our community, the journey with an ultra-rare disease begins with a question mark. It’s a path that often involves a long, frustrating search for answers—a “diagnostic odyssey”—and leads to a life of managing complex symptoms without a therapy that addresses the root of the problem. We know this journey can be incredibly challenging, and we want you to know that on this path, you are not alone.
Today, we want to walk with you through a story of incredible scientific progress that brings a new chapter of hope for one such condition: WHIM syndrome.
This is a deep dive into the science, the challenges, and the landmark progress that has led to the first-ever therapy designed specifically for this rare disorder. We believe that by understanding the journey from discovery to treatment, we can have more informed conversations, ask better questions, and march forward together with a clearer sense of what the future holds for our communities.
What is WHIM Syndrome? A Story in an Acronym
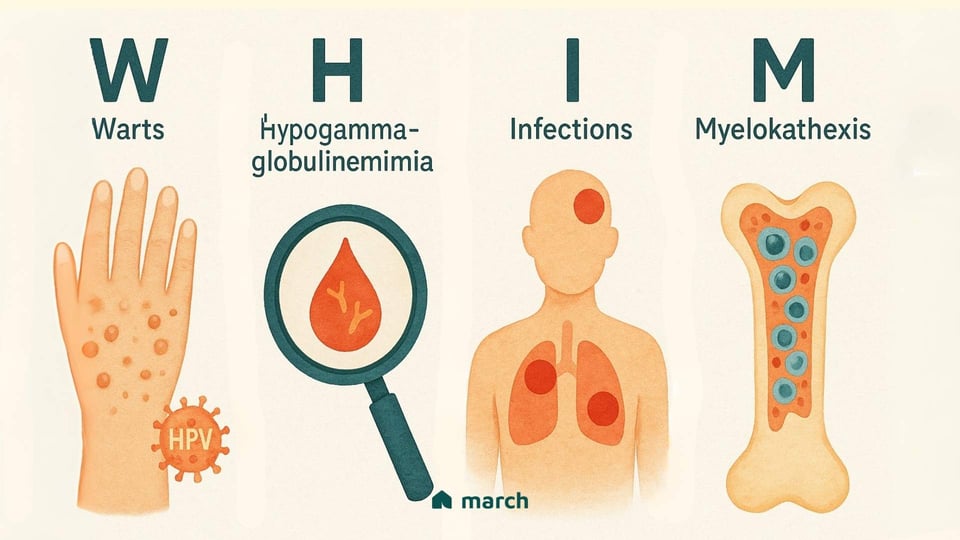
WHIM syndrome is an extremely rare, genetic primary immunodeficiency, and its name is an acronym that tells the story of its main features:[1]
-
Warts: Patients often experience severe, persistent, and widespread warts caused by an unusual susceptibility to the human papillomavirus (HPV).
-
Hypogammaglobulinemia: This means having low levels of immunoglobulins (antibodies) in the blood, which are the body’s essential tools for fighting off bacteria and other germs.
-
Infections: The combination of immune system challenges leads to recurrent and often serious bacterial infections, such as pneumonia, sinusitis, and skin infections.
-
Myelokathexis: This is the scientific term for the unusual and defining feature of WHIM. It describes a situation where the body’s key infection-fighting white blood cells, particularly neutrophils, get "trapped" in the bone marrow and can’t get out into the bloodstream to do their job.[2]
Imagine your body’s best soldiers are all trained and ready for battle, but they’re stuck behind a locked door in the barracks, unable to get to the front lines.[3] This is the core problem of WHIM syndrome. The bone marrow is packed with healthy, mature immune cells, but the rest of the body is left vulnerable.
The Root of the Challenge: An Overactive "Doorbell" in the Immune System
So, what’s keeping that door locked? For decades, this was a mystery. But in 2003, researchers made a breakthrough discovery: WHIM syndrome is caused by a mutation in a single gene called CXCR4.[4]
This gene provides the instructions for a receptor that acts like a cellular “doorbell.” It sits on the surface of white blood cells and its main job is to receive a signal from a molecule called CXCL12. This signal essentially tells the white blood cells to stay within the safe, nurturing environment of the bone marrow.[5]
In a healthy person, this doorbell rings, the cell gets the "stay put" message, and then the doorbell quickly turns off. This allows the cells to leave the bone marrow when they’re called to action to fight an infection.
In WHIM syndrome, the mutation causes a “gain-of-function.” This means the CXCR4 doorbell is overactive—it’s essentially stuck in the “on” position.[6] It constantly signals for the cells to stay put, even when there's no active signal telling them to.
This is the direct cause of Myelokathexis. The relentless "stay put" signal from the faulty CXCR4 receptor is what locks the door, trapping mature neutrophils and lymphocytes in the bone marrow. There, they eventually grow old and die without ever reaching the parts of the body that desperately need them. This single genetic typo is what leads to the low white blood cell counts (neutropenia and lymphopenia) that cause the recurrent infections and other symptoms of WHIM.
The Journey to a Solution: From Managing Symptoms to Targeting the Cause
For decades, managing WHIM syndrome meant treating the consequences of the locked door, not the lock itself. This supportive care has been, and continues to be, a vital lifeline for patients.
Granulocyte-Colony Stimulating Factor (G-CSF) injections can help push more neutrophils out of the bone marrow, but this treatment doesn’t address the underlying problem with other immune cells and can come with long-term risks.[1]
Immunoglobulin (IVIG) infusions can provide the body with borrowed antibodies to help fight infections, but this is a temporary fix that requires lifelong, burdensome infusions.
These are incredibly important tools, but they are “workarounds.” They don’t fix the faulty doorbell. The community has long needed a therapy that could directly address the root genetic cause of the disease.
A Landmark Moment: The Arrival of Mavorixafor (XOLREMDI®)
After years of dedicated research, a landmark moment arrived. In April 2024, the U.S. FDA approved mavorixafor (brand name XOLREMDI®), the first and only therapy designed specifically to target the underlying mechanism of WHIM syndrome for patients aged 12 and older.[7]
Let's return to our analogy. If the problem is an overactive doorbell, mavorixafor works by temporarily putting a cover over it.
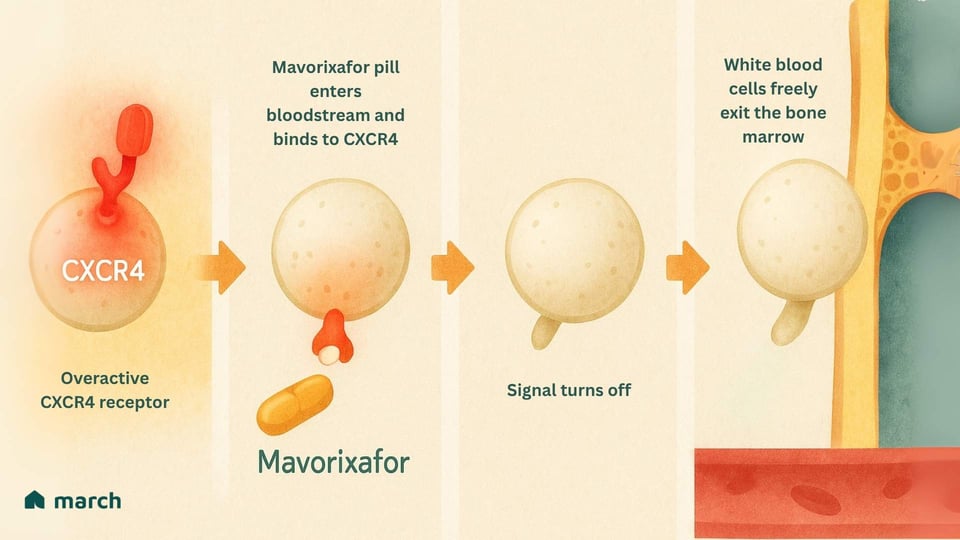
Mavorixafor is an oral medication that is a CXCR4 antagonist. This means it binds to the CXCR4 receptor and blocks it, preventing it from constantly sending its "stay put" signal.[8]
By interrupting this signal, it essentially “unlocks the door.” The trapped neutrophils and lymphocytes are finally released from the bone marrow and can enter the bloodstream to patrol for infections and protect the body.[7]
The results from the pivotal Phase 3 clinical trial were a source of profound hope for the community. Patients taking mavorixafor saw:[9]
-
A statistically significant increase in the amount of time their neutrophil and lymphocyte counts were above the levels needed to fight infection.
-
Most powerfully, a 60% reduction in the rate of severe infections compared to those taking a placebo.
This represents a monumental step forward—a shift from simply managing the fallout of the disease to actively intervening at its source.
Marching Forward Together: Hope, Realism, and the Journey Ahead
The approval of a targeted therapy for an ultra-rare disease is a victory for the entire rare disease community. It’s a testament to the resilience of the patients and families who participate in clinical trials, and to the dedication of the researchers and advocates who champion progress.
At the same time, as a community, we walk this path with both hope and honesty. This is a huge step, but it’s not the end of the journey. In its main trial, the therapy didn’t show a significant improvement in the burden of warts, and its approval is currently for patients 12 and older, which highlights an ongoing need for research in younger children.[9,10] Mavorixafor is a daily, lifelong management therapy, not a one-time cure, and it requires careful adherence.
This is what the forward march of progress looks like. It is a dedicated, meaningful step that can significantly reduce the burden of infections and transform the way a disease is managed. And it inspires the next wave of innovation. The remarkable story of a patient who was spontaneously cured of WHIM when a genetic accident deleted her faulty CXCR4 gene has provided a natural proof-of-concept for a potential cure, sparking research into gene therapies that could one day replicate that outcome intentionally.[11]
As we watch these new frontiers of medicine unfold, we are committed to providing you with clear, trustworthy information. We will celebrate the breakthroughs and be honest about the challenges that remain. We will continue to build a community where we can learn, share, and support one another. Your insights, your experiences, and your hope are what fuel this forward march. On this journey of discovery, you are not alone.
For a quick overview of another scientific breakthrough, listen to our latest podcast episode, which delves into the history and science behind new gene editing treatments.
Sources
[1] National Organization for Rare Disorders (NORD). (n.d.). WHIM Syndrome.
[2] Zuelzer, W. W. (1964). "Myelokathexis"--a new form of chronic granulocytopenia. New England Journal of Medicine.
[3] Dale, D. C., et al. (2020). The CXCR4 antagonist mavorixafor in patients with WHIM syndrome. Blood.
[4] Hernandez, P. A., et al. (2003). Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nature Genetics.
[5] X4 Pharmaceuticals. (2024). XOLREMDI (mavorixafor) Prescribing Information.
[6] Balabanian, K., et al. (2005). WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization and internalization. Blood.
[7] Badalato, R., et al. (2024). Mavorixafor for treatment of WHIM syndrome: a phase 3, randomised, placebo-controlled trial. The Lancet.
[8] McDermott, D. H., et al. (2019). The CXCR4 antagonist plerixafor corrects panleukopenia and prevents bacterial infections in WHIM syndrome. Blood.
[9] X4 Pharmaceuticals. (2024, April 29). X4 Pharmaceuticals Announces U.S. FDA Approval of XOLREMDI™ (mavorixafor). [Press Release].
[10] U.S. Food & Drug Administration (FDA). (2024). Novel Drug Approvals for 2024.
[11] McDermott, D. H., et al. (2015). Chromothriptic cure of WHIM syndrome. Cell.










Sadaf boostan
•3 months, 4 weeks ago
Great
Report Comment
You are about to report:
Sadaf boostan
sadaf sadaf
•3 months ago
Awesome
Report Comment
You are about to report:
sadaf sadaf