For so many families in our community, the journey with Hemophilia B has been one of resilience, strength, and constant vigilance. It’s a path measured in infusions, marked by the ever-present need to manage a condition that works from deep within the body’s instruction manual. We know the weight of this journey—the planning, the needle sticks, and the worry about the bleeds that can disrupt life without warning. The standard of care, life-saving as it is, has always been a lifelong commitment.
The collective dream has always been for something more. For a treatment that could offer freedom, reduce the burden, and address the condition at its very source. Today, we want to walk together through the story of a monumental leap in that direction: gene therapy.
It’s a field brimming with incredible promise, but also one of complex science and real-world challenges. By taking a deep and honest look at the journey of a specific therapy, we can better understand the hope that lies on the horizon and the hurdles we, as a community, must navigate together. This is a story of science, of hope, and of the unshakeable spirit of a community marching forward.
Understanding Our Shared Journey with Hemophilia B
Before we can appreciate where we’re going, it’s important to honor where we are. Hemophilia B, which some may know by its historical name "Christmas Disease," is a rare genetic disorder that affects the blood's ability to clot properly. This happens because of a defect in a single gene, the F9 gene, which holds the instructions for making a vital clotting protein called Factor IX (FIX).
This gene is located on the X chromosome, which is why hemophilia primarily affects boys and men. A male inherits one X chromosome from his mother, and if that chromosome carries the altered F9 gene, he will have Hemophilia B. It’s important to remember that this isn't always inherited; about one-third of cases happen because of a new, spontaneous change in the gene, meaning there was no previous family history.
A Spectrum of Experience
We know that every journey is different, and this is especially true for Hemophilia B. The severity of the condition depends entirely on how much functional Factor IX a person’s body can produce.
-
Severe Hemophilia B (<1% FIX activity): For those with the severe form of the condition, life involves the risk of frequent, spontaneous bleeding into joints and muscles without any obvious injury. This can start in early infancy and requires constant management to prevent long-term, painful joint damage.
-
Moderate Hemophilia B (1-5% FIX activity): Here, spontaneous bleeds are less common, but bleeding after minor injuries, dental work, or surgery can be prolonged and serious.
-
Mild Hemophilia B (6-40% FIX activity): Individuals with mild Hemophilia B may only experience bleeding problems after a major accident or surgery, and some may not even be diagnosed until later in life.
No matter the severity, the impact on life is real. The hallmark of hemophilia is bleeding into joints—the knees, elbows, and ankles—which causes pain, swelling, and, over time, can lead to irreversible damage known as hemophilic arthropathy. This is why the conversation in our community is so often focused not just on treating bleeds, but on preventing them.
This shared understanding—the desire to protect our children and ourselves from the long-term consequences of this condition—is the very reason the search for better treatments has been so relentless.
The Path We've Traveled—A History of Progress and Resolve
To understand the excitement around gene therapy, we have to look back at the incredible, and sometimes painful, road our community has traveled. The history of hemophilia treatment is a powerful story of medical progress, transforming a condition that was once a childhood death sentence into a manageable, chronic one.
In the early 20th century, life expectancy was tragically short. The first real breakthrough came from understanding that something in blood plasma could help clotting. This led to infusions of plasma, and later, a concentrate called cryoprecipitate, which was richer in clotting factors.
The 1970s brought a revolution: freeze-dried factor concentrates. For the first time, families could store the treatment at home and infuse it intravenously at the first sign of a bleed. This empowered patients, gave them independence, and dramatically reduced hospital visits. It also paved the way for the new standard of care: prophylaxis, the regular, scheduled infusion of factor to prevent bleeds from ever happening. This proactive approach has been instrumental in preserving joint health and allowing so many in our community to live fuller, more active lives.
A Shadow on the Progress
But this era of progress was marked by a devastating tragedy. In the 1980s, the blood supply from which these factor concentrates were made became contaminated with HIV and Hepatitis C. This crisis was a profound blow, infecting an estimated half of the entire hemophilia patient population in the U.S. with HIV and causing immense suffering and loss.
This dark chapter, however, also galvanized our community. It spurred a powerful, unified demand for safer treatments and accelerated the development of the next great leap: recombinant factors. These were clotting factors made using genetic engineering, completely free from human plasma and the risk of blood-borne viruses. The first recombinant Factor IX was approved in 1997.
From standard-acting recombinant factors to the newer Extended Half-Life (EHL) products that allow for less frequent infusions, the goal has always been the same: to make treatment safer, more effective, and less burdensome. But even with these incredible advances, the need for lifelong, regular IV infusions remained.
This entire journey—from the earliest discoveries to the hard-won fight for safer products—set the stage for the next logical, hopeful question: What if, instead of just replacing the factor, we could empower the body to make its own?
A New Chapter of Hope—The Science of Gene Therapy
For years, this question was the dream that fueled labs and researchers around the world. Today, it’s a scientific reality. Let's break down the science of gene therapy for Hemophilia B in a way that feels approachable.
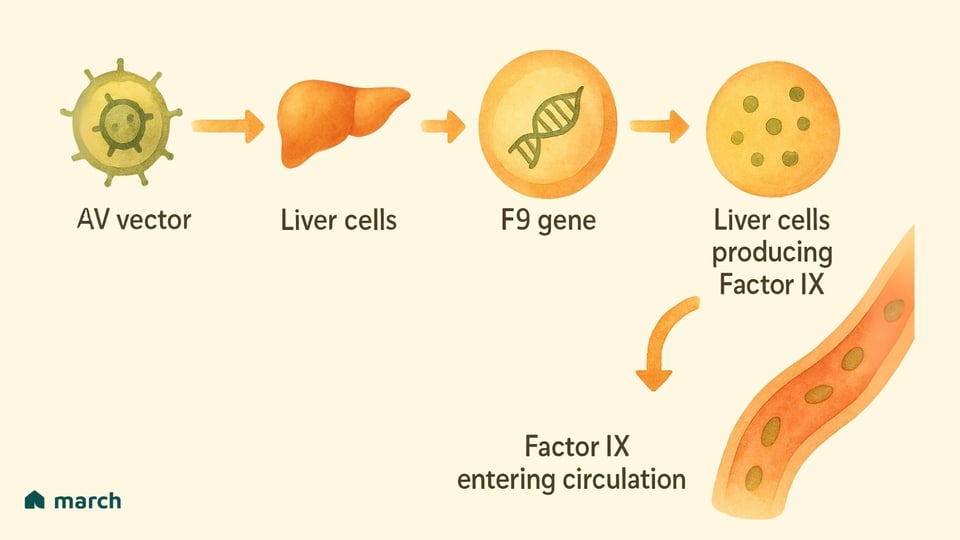
At its heart, the strategy for Hemophilia B is gene replacement. The goal is to deliver a new, functional copy of the F9 gene into the body’s cells, turning them into factories for producing their own Factor IX.
Think of it like a special delivery service:
-
The Delivery Truck: Scientists use a harmless, re-engineered virus called an Adeno-Associated Virus (AAV) as the delivery vehicle. This virus has been chosen because it doesn’t cause disease in humans, and for gene therapy, its own genes are removed so it can't replicate. It’s simply a carrier.
-
The Package: Inside this AAV truck is the precious cargo: a working copy of the human F9 gene.
-
The Destination: The therapy is given as a one-time intravenous (IV) infusion. The AAV vectors then travel through the bloodstream to their primary destination: the liver. This is strategic, because the liver is the body's natural site for producing clotting factors.
Once the AAV vector delivers its genetic package to the liver cells, those cells can start using the new instructions to produce and release their own Factor IX into the bloodstream, hopefully for a very long time. It’s a remarkable approach that aims to provide a continuous, internal supply of the very protein that’s missing.
A Case Study in Smart Science: Fidanacogene Elaparvovec (Beqvez)
One of the therapies that brought this science to life is called fidanacogene elaparvovec, which you may know by its brand name, Beqvez. Its development journey, rooted in decades of research, showcases how scientists have worked to make this approach even better.
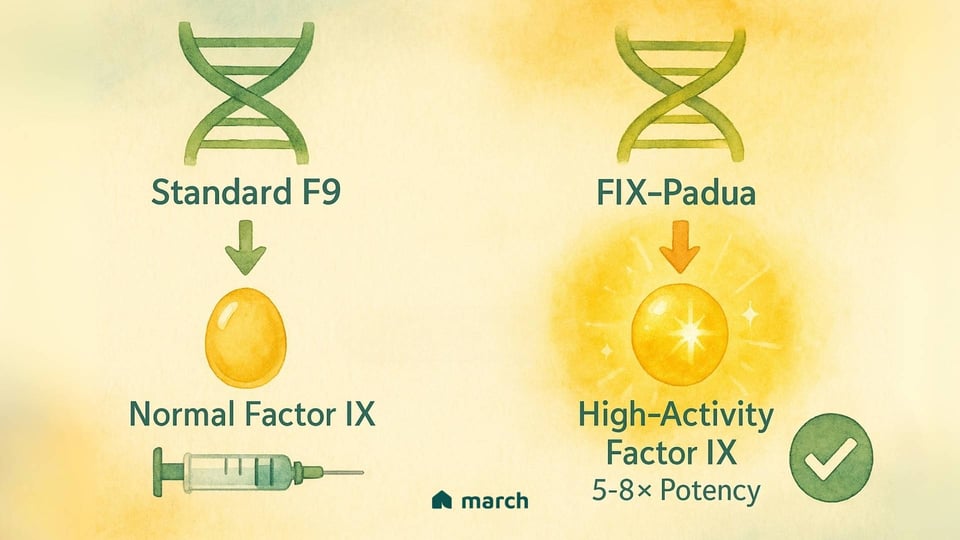
The team behind Beqvez incorporated a truly clever innovation into its design. Instead of using a standard F9 gene, they used a naturally occurring, high-activity variant called FIX-Padua. This version of the gene produces a Factor IX protein that is about five to eight times more potent than the normal protein.
This was a brilliant move. By using a gene that codes for a "supercharged" protein, the body doesn't need to produce as much of it to get a powerful, protective effect. This, in turn, meant that a lower dose of the AAV "delivery truck" could potentially be used, which is always a key goal when thinking about safety. It’s a perfect example of how science marches forward, constantly refining and improving.
The Story of Beqvez—A Lesson in Hope and Hurdles
When a new therapy moves into human clinical trials, it's a moment our entire community watches with bated breath. The pivotal Phase 3 trial for Beqvez, called BENEGENE-2, provided a wealth of information about its potential.
The results offered a profound sense of hope. For the men with moderately severe to severe Hemophilia B who participated, a single infusion of Beqvez led to remarkable outcomes:
-
A Dramatic Drop in Bleeds: The mean annualized bleeding rate (ABR) fell by 71% compared to when they were on their regular prophylaxis.
-
Zero Bleeds for Most: Perhaps most powerfully, 60% of the participants experienced zero bleeding episodes in the year following treatment.
-
Freedom from Prophylaxis: After treatment, their bodies were producing Factor IX at levels in the mild hemophilia or even normal range, allowing them to stop their burdensome routine infusions.
These aren't just statistics; they represent a life-changing shift. The possibility of living without the constant need for infusions and with greatly reduced fear of bleeding is the very dream we’ve all been holding onto.
The Hurdles on the Path
As a community, we know that the path of progress is rarely a straight line. We walk this road holding both hope and realism, and the story of Beqvez also includes significant challenges.
One of the biggest hurdles for AAV-based gene therapies is pre-existing immunity. Because AAVs are naturally occurring, many of us have been exposed to them and have developed antibodies. If a person has these antibodies, their immune system may block the AAV "delivery truck" before it can do its job, making the therapy ineffective. This is why patients must be tested for these specific antibodies before receiving treatment.
The other major challenge is the one that ultimately changed the course for Beqvez: the complex reality of market access. Despite its scientific success and approvals from regulatory bodies like the FDA, Pfizer, the company behind Beqvez, decided to halt its commercialization globally in early 2025. The reasons cited were not related to safety or how well the therapy worked. Instead, they were due to "commercial reasons," including limited uptake from patients and doctors and the challenges of fitting a high-cost, one-time therapy ($3.5 million per dose) into our existing healthcare and reimbursement systems.
This can be difficult news to process. It's a powerful reminder that even the most brilliant science must find a sustainable path through the practical and financial realities of the world. It shows that our advocacy as a community—for innovative payment models and equitable access—is just as important as the research itself.
The Horizon Ahead—More Than One Path Forward
The story of Beqvez is not an ending. It is a vital chapter in an ongoing narrative of progress, and the knowledge gained will continue to light the way forward. It's crucial to remember that there is more than one path on this new horizon.
Other Gene Therapy Options
Another gene therapy, etranacogene dezaparvovec (Hemgenix), was the first to be approved for Hemophilia B in the U.S. It works in a very similar way, using an AAV vector to deliver the same potent FIX-Padua gene to the liver. The availability of more than one option is a positive sign, showing that the field is robust and continuing to advance.
A Different Approach: Rebalancing the System
Perhaps just as exciting is an entirely different class of therapies emerging that don’t involve replacing Factor IX at all. These are often called "rebalancing agents." They work by targeting the body’s natural anticoagulants—the proteins that act as the "brakes" on the clotting system.
By gently easing up on these brakes, therapies like concizumab (Alhemo), which targets a protein called TFPI, can help rebalance the system and make it easier for the body to form a clot. Another investigational therapy, fitusiran, works by targeting a different brake called antithrombin.
These approaches are so important for several reasons:
-
Convenience: They are typically given as a subcutaneous injection (under the skin), which is much less invasive than IV infusions.
-
A Solution for Inhibitors: One of the most serious challenges in hemophilia care is when the body develops antibodies, or "inhibitors," against replacement factor, making treatment ineffective. Because these rebalancing therapies don't use Factor IX, they can be an effective option for this very difficult-to-treat group.
Marching Forward, Together
The landscape of Hemophilia B treatment is changing faster than ever before. We are moving from an era of simply managing the condition to one where we can envision fundamentally altering it. This journey is filled with complex science, personal decisions, and real-world hurdles.
As a community, our role is to continue to learn, to ask questions, and to support one another. We will celebrate the incredible breakthroughs and be honest and realistic about the challenges. We will advocate for a future where these life-changing innovations are accessible to everyone who needs them.
Your journey, your experiences, and your hope are the fuel for this forward march. We are on this path of discovery together, and on this path, you are not alone.
Sources
National Bleeding Disorders Foundation. (n.d.). "Prophylaxis." Retrieved from https://www.hemophilia.org/bleeding-disorders-a-z/treatment/prophylaxis
Centers for Disease Control and Prevention (CDC). (2023, August 15). "What is Hemophilia?" Retrieved from https://www.cdc.gov/ncbddd/hemophilia/facts.html
National Organization for Rare Disorders (NORD). (n.d.). "Hemophilia B." Retrieved from https://rarediseases.org/rare-diseases/hemophilia-b/
MedlinePlus. (n.d.). "Hemophilia." Retrieved from https://medlineplus.gov/genetics/condition/hemophilia/
World Federation of Hemophilia. (n.d.). "Severity of Hemophilia." Retrieved from https://elearning.wfh.org/elearning-centres/hemophilia-a-and-b/severity-of-hemophilia/
National Bleeding Disorders Foundation. (n.d.). "Joint Damage (Arthropathy)." Retrieved from https://www.hemophilia.org/bleeding-disorders-a-z/complications/joint-damage-arthropathy
Hoglund, P., & Wolberg, A. S. (2022). "The history of hemophilia: A bloody long story." Journal of Thrombosis and Haemostasis. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9313885/
Hemophilia Federation of America. (n.d.). "History of Bleeding Disorders." Retrieved from https://www.hemophiliafed.org/understanding-bleeding-disorders/history-of-bleeding-disorders/
The New York Times. (1993, November 13). "Blood, Money and AIDS: Hemophiliacs Are Split; Files Suggest Maker of Clotting Drug Knew of AIDS Risk." Retrieved from https://www.nytimes.com/1993/11/13/us/blood-money-aids-hemophiliacs-are-split-files-suggest-maker-clotting-drug-knew.html
U.S. Food and Drug Administration (FDA). (1997, February 25). "BeneFIX - Approval letter." Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/appletter/1997/961183ltr.pdf
National Bleeding Disorders Foundation. (n.d.). "Gene Therapy." Retrieved from https://www.hemophilia.org/bleeding-disorders-a-z/treatment/gene-therapy
American Society of Gene & Cell Therapy. (n.d.). "AAV Vectors." Retrieved from https://patienteducation.asgct.org/gene-therapy-101/aav-vectors
U.S. Food and Drug Administration (FDA). (2024, April 26). "FDA Approves First Gene Therapy for Adults with Moderate to Severe Hemophilia B." Retrieved from https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-adults-moderate-severe-hemophilia-b-0
George, L. A. (2017, December 7). "Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant." New England Journal of Medicine. Retrieved from https://www.nejm.org/doi/full/10.1056/nejmoa1708538
Pfizer Inc. (2024, April 26). "U.S. FDA Approves Pfizer’s BEQVEZ™ (fidanacogene elaparvovec-dzkt), a One-Time Gene Therapy for Adults with Moderate to Severe Hemophilia B." Retrieved from https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-beqveztm-fidanacogene-elaparvovec
Kansteiner, F. (2025, February 28). "Pfizer to pull the plug on hemophilia B gene therapy Beqvez." Fierce Pharma. Retrieved from https://www.fiercepharma.com/pharma/pfizer-pull-plug-hemophilia-b-gene-therapy-beqvez
Taylor, P. (2024, April 29). "Pfizer matches price of CSL’s Hemgenix with $3.5M for Beqvez." PharmaPhorum. Retrieved from https://pharmaphorum.com/news/pfizer-matches-price-csls-hemgenix-35m-beqvez
U.S. Food and Drug Administration (FDA). (2022, November 22). "FDA Approves First Gene Therapy to Treat Adults with Hemophilia B." Retrieved from https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treat-adults-hemophilia-b
National Bleeding Disorders Foundation. (n.d.). "Non-Factor Therapies." Retrieved from https://www.hemophilia.org/bleeding-disorders-a-z/treatment/non-factor-therapies
Novo Nordisk. (2024, March 19). "Novo Nordisk receives US FDA approval for Alhemo® (concizumab) for the treatment of haemophilia A and B with inhibitors." Retrieved from https://www.novonordisk.com/news-and-media/news-and-ir-materials/news-details.html?id=167098
Sanofi. (2024, March 4). "Pivotal Phase 3 data on fitusiran, an investigational siRNA therapy for hemophilia A or B, with or without inhibitors, published in The Lancet." Retrieved from https://www.sanofi.com/en/media-room/press-releases/2024/2024-03-04-14-00-00-2834371
Centers for Disease Control and Prevention (CDC). (2023, August 15). "Inhibitors." Retrieved from https://www.cdc.gov/ncbddd/hemophilia/inhibitors.html










